The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a) pressure p1 and (b) temperature T2? 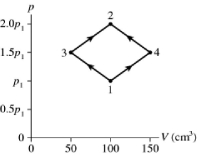
Definitions:
Carbon Dioxide
A colorless, odorless gas produced by burning carbon and organic compounds and by respiration, often considered a greenhouse gas contributing to global warming.
Bicarbonate Ions
Chemical ions (HCO3-) that act as a buffering agent in blood, maintaining pH balance by neutralizing acids.
Carbonic Acid
A weak acid that forms when carbon dioxide dissolves in water, playing a crucial role in the carbon dioxide transport of blood and the acid-base balance in the body.
Thoracic Cavity
The part of the body located between the neck and the abdomen, housing the heart and lungs within the rib cage.
Q10: How much work must be done
Q24: A 0.24 kg blob of clay
Q48: A 0.14-kg baseball is dropped from rest
Q50: A ball is oscillating on an ideal
Q53: A fire hose is turned on the
Q74: When a car of mass
Q80: A 475-gram ball is traveling horizontally at
Q95: A 0.140-kg baseball is dropped and reaches
Q146: A toy rocket that weighs 10 N
Q173: Which one of the following quantities is