The figure shows a pV diagram for 2.9 g of ideal oxygen gas O2 in a sealed container. The temperature of state 1 is 76° C, the atomic mass of the oxygen atom is 16 g/mol, and R = 8.31 J/mol ∙ K. What are the temperatures T3 and T4? 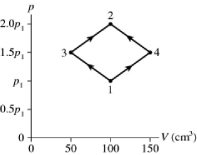
Definitions:
Greatest Common Monomial Factor
The highest monomial that divides evenly into each term of an algebraic expression.
Factor By Grouping
A method for factoring polynomials that involves grouping terms with common factors and then factoring out those common factors.
Common Binomial Factor
A binomial that is a factor of two or more polynomials.
Prime Factors
Prime factors of a number are the prime numbers that divide the given number exactly, without leaving a remainder.
Q4: What is the net power needed to
Q11: Heat is added to a 3.0
Q18: The figure shows a graph of the
Q54: The small piston of a hydraulic lift
Q66: Two horizontal pipes are the same
Q81: A dinner plate falls vertically to the
Q92: A sealed rigid tank contains 29
Q96: A hot air balloon along with its
Q172: A stone is held at a height
Q202: In a given reversible process, the temperature