One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO₃) . Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO₃⁻) and a hydrogen ion (H⁺) . (See figure.) 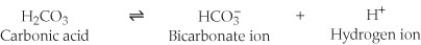
If the pH of blood drops, one would expect ________.
Definitions:
French Colonialism
The period of expansion, conquest, and administration by France over various territories across the globe, notably in Africa and Southeast Asia.
Marshall Plan
An American initiative passed in 1948 to aid Western Europe, in which the United States gave over $12 billion (nearly $100 billion in 2020 dollars) in economic assistance to help rebuild Western European economies after the end of World War II.
Objectives
Represents goals or targets that are intended to be achieved in a specific period.
United States
A country in North America consisting of 50 states, known for its significant influence in global economics, culture, and politics.
Q5: Amoebae move by crawling over a surface
Q11: Cyanide binds with at least one molecule
Q17: The synthesis of ATP by oxidative phosphorylation,
Q20: Companies design _ to embody or express
Q40: Starting with one molecule of glucose, glycolysis
Q50: Exposing inner mitochondrial membranes to ultrasonic vibrations
Q55: Use the figures to answer the question.<br><img
Q61: A clique is a small group whose
Q65: Which of the following frequently imposes a
Q70: Volkswagen's famed "Drivers Wanted" advertising campaign aimed