Which statement A-D regarding the reaction described by the energy profile below is not correct? 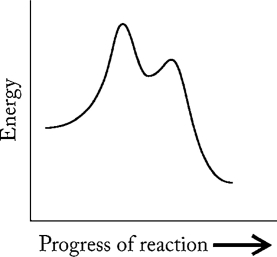
Definitions:
Poisson Distribution
A statistical distribution that expresses the probability of a given number of events occurring in a fixed interval of time or space, assuming these events occur with a known constant rate and independently of the time since the last event.
M/M/1 Queue
A model describing a queueing scenario with a single server, where arrivals are determined by a Poisson process, and service times have an exponential distribution.
Units in the System
The total number of items or entities currently being processed or within a particular process or system at any given time.
Poisson Distribution
A statistical distribution describing the probability of a given number of events happening in a fixed interval of time or space, assuming events happen independently at a constant rate.
Q57: Ammonia (NH<sub>3</sub>) acts as a weak base
Q73: For the chemical equilibrium aA +bB
Q82: Many metal cations produce aqueous solutions that
Q95: Determine the order of the reaction
Q97: Three common weak bases are phosphate (P;
Q100: The freezing point of a 0.060 m
Q115: The vapor pressure of an aqueous solution
Q147: The linear form of the Arrhenius equation
Q165: Which of the following compounds would not
Q167: Bert and Ernie were determining the pH