In the diagram below, one beaker contains pure water and the other contains an equal volume of seawater. Seawater has various salts dissolved in it. The beakers are sitting in a totally enclosed chamber, and the outside temperature and pressure are held constant. Identify the statements below about this situation that are not correct. 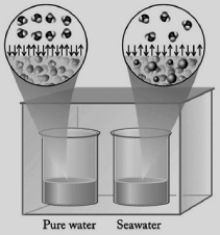 I. Water will be transferred from the pure water beaker to the seawater beaker.
I. Water will be transferred from the pure water beaker to the seawater beaker.
II) The vapor pressure of the seawater is higher than the vapor pressure of the pure water.
III) Pure water evaporates at a faster rate than seawater.
IV) Water in the gas phase condenses into the seawater beaker at a faster rate than into the pure water beaker.
Definitions:
Q13: A cubic closest-packed structure has hexagonally arranged
Q15: Draw a structure showing the geometry of
Q24: Which of the following molecules has a
Q30: The correct H-N-H bond angle in
Q33: Cyclic alkanes differ from normal alkanes in
Q34: If a face-centered cubic unit cell has
Q34: The freezing point of a 0.0925 m
Q47: The reaction 2NO(g) + O<sub>2</sub>(g)
Q76: Which statement about the shape or geometry
Q105: Which statement about VSEPR theory is not