What is the vapor pressure of an aqueous solution that has a solute mol fraction of 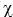 = 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
= 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
Definitions:
Open Door Policy
A foreign policy principle proposed by the United States in the late 19th and early 20th centuries advocating for equal trading rights among nations in China.
Imperialist Powers
Countries that extend their power and influence through colonization, use of military force, or other means.
Nationalist Leader
A person who promotes the interests, culture, or independence of their nation, sometimes leading a movement or party.
Emilio Aguinaldo
A Filipino revolutionary, politician, and military leader who is considered to be the first and the youngest President of the Philippines and led Filipino forces during the Spanish-American War and the Philippine-American War.
Q3: Boiling points increase in the order HCl
Q3: Which of the following is an amine
Q16: The energy profiles for four different reactions
Q17: Which hydride do you predict has the
Q18: For a chemical reaction at equilibrium, which
Q20: Which one of the ionic compounds below
Q21: The reaction of bromine gas with
Q28: Which one of the following molecules violates
Q90: The concentration unit of molality is symbolized
Q91: What is the formal charge of the