What is the vapor pressure of an aqueous solution that has a solute mol fraction of 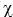 = 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
= 0.100? The vapor pressure of water is 25.756 mm Hg at 25°C.
Definitions:
Enthalpy Reaction
A thermodynamic quantity equivalent to the total heat content of a system, representing the sum of its internal energy and the product of its pressure and volume, especially useful in the study of heat exchange in chemical reactions.
Electrochemical Reaction
A chemical reaction that involves the transfer of electrons from one species to another, often associated with electricity.
Exergonic
Describing a chemical reaction that releases energy to its surroundings, typically in the form of heat or work.
Endergonic
Endergonic refers to a chemical reaction where energy is absorbed from the surroundings, generally requiring an input of energy to proceed.
Q10: A mixture of salt and ice is
Q34: Which of the following compounds has the
Q40: In a catalyzed reaction, the _ of
Q40: Nitrite (NO<sub>2</sub><sup>-</sup>) is an important nutrient in
Q55: The linear form of the Arrhenius equation
Q60: The higher the carbon content in steel,
Q69: In which of the following molecules does
Q78: Which of the following molecules contains an
Q103: How many valence electrons does S<sup>2</sup><sup>-</sup> have?<br>A)
Q136: For the rate law Rate = k[A][B]<sup>3/2</sup>,