Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below. Explain why you think this molecule is planar or nonplanar. 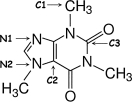
Definitions:
Racial Profiling
The unfair and prejudicial treatment of individuals based on their race or ethnicity, often manifested in unjust scrutiny or assumptions of guilt.
Two-Factor Theory
A psychological theory that proposes that emotional experience is the result of a combination of physiological arousal and cognitive interpretation.
Reciprocity Norm
A social norm that expects individuals to help those who have helped them, fostering mutual support and cooperation.
Ingroup Bias
The tendency to favor one's own group over other groups.
Q9: Hydrophilic substances _<br>A) are immiscible in water.<br>B)
Q10: When a certain acid and a certain
Q35: Which statement about refraction or diffraction is
Q65: The term colligative refers to properties that
Q81: Ethanol has the formula CH<sub>3</sub>CH<sub>2</sub>OH. The central
Q94: Which set of gases is listed from
Q111: Which molecule has a stretching vibration that
Q123: Dry air consists of 78.1% nitrogen (28
Q124: The temperature at point a in the
Q126: Indicate which of the following molecules has