According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO+, and NO-? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 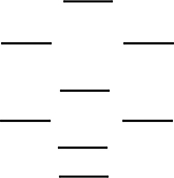
Definitions:
Identity
The distinct characteristics, personality, or qualities that make an individual or group unique.
Quantity Theory of Money
A theory that relates the money supply to the price level and the rate of economic growth.
Money Supply
The sum of all financial assets, encompassing cash and deposits in banks, present within an economy at a given moment.
Price Level
An indicator that measures the typical prices for a comprehensive selection of goods and services within the economy.
Q1: Different structural forms of the elements are
Q10: In quantum mechanics, an atomic orbital _<br>A)
Q20: Which one of the ionic compounds below
Q49: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q50: Which alcohol should be most soluble in
Q62: What is the geometry of the ClF<sub>4</sub><sup>-</sup>
Q72: A 25.0 L balloon is filled with
Q88: Electrical and thermal conductivity in metals _<br>A)
Q90: The concentration unit of molality is symbolized
Q118: The van der Waals equation for real