What wavelength of light is required to cause the ejection of photoelectrons with kinetic energies of 5.0 *10-20 J from a calcium surface (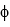 = 4.60 * 10-19 J) ?
= 4.60 * 10-19 J) ?
Definitions:
Bonds Payable
Bonds payable refers to the amount of money a company owes to holders of its bonds, representing a long-term debt obligation.
Credit Cash
An accounting entry that represents an increase in cash or cash equivalents, indicating funds being added to the company’s assets.
Bond Interest Expense
The cost incurred by an issuer of bonds for the interest required to be paid to bondholders.
Contract Rate
The agreed-upon rate of interest or payment terms specified within a financial contract or agreement.
Q1: How many moles of an ideal gas
Q11: The Fraunhofer lines are evidence that _<br>A)
Q44: Which statement provides the best description of
Q59: A 5 L diving tank is filled
Q81: Which of the following compounds would have
Q102: Arrange the following compounds in order of
Q106: Which statement A-D about the wave
Q127: A 15 g piece of iron (C<sub>P</sub>
Q133: A balloon is filled with 3.00 L
Q137: What color will a blue object appear