What wavelength of light is required to cause the ejection of photoelectrons with kinetic energies of 5.0 *10-20 J from a calcium surface (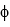 = 4.60 * 10-19 J) ?
= 4.60 * 10-19 J) ?
Definitions:
Utility Functions
Mathematical models that represent preferences over a set of goods or outcomes, used in economics to understand decision making.
Peak Performance
Achieving the highest level of performance capability, often characterized by exceptional focus, skill, and determination.
Validity Problem
An issue that arises when the measure or test does not accurately capture or represent what it is intended to assess.
Performance Measurement
The process of evaluating the efficiency and effectiveness of an action or organization through quantifiable indicators.
Q42: How many orbitals that have the principal
Q43: Which electron-pair geometry corresponds to a steric
Q51: How many valence electrons surround the xenon
Q80: Electromagnetic radiation with a frequency of 8.6
Q80: Which is the dominant interaction between oxygen
Q92: Early photoelectric light detectors were based on
Q114: Which of the following molecules has a
Q115: Which one of the following statements is
Q115: In which bond does the H atom
Q130: Which type of molecular orbital contains