The pH of an acidic solution is a measure of the concentration of the acid particles in the solution, with smaller values of the pH indicating higher acid concentration. In a laboratory experiment, the pH of a certain acid solution is changed by dissolving over-the-counter antacid tablets into the solution. In this experiment, the pH changes according to the equation 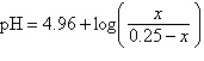 , where x is the number of grams of antacid added to the solution. What is the pH of the solution after the addition of 0.1 grams of antacid tablet?
, where x is the number of grams of antacid added to the solution. What is the pH of the solution after the addition of 0.1 grams of antacid tablet?
Definitions:
Overlapping Systems
Intersecting social categorizations such as race, class, and gender, affecting an individual's or group's experience and opportunities.
Class Position
The rank or standing of an individual within the hierarchy of social class, often determined by factors like wealth, occupation, and education.
Stratification
The arrangement or classification of something into different groups or layers, often based on socioeconomic status.
Wealth
The monetary value of everything one actually owns.
Q11: owner of a convertible bond owns, in
Q12: Speculative risks are symmetrical in the sense
Q16: Refer to Figure 9-3.<br>A) Firm X is
Q19: Discounted cash flow methods are not appropriate
Q22: Although goodwill created in a merger may
Q29: Determine whether the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4588/.jpg" alt="Determine
Q40: During one performance of a local arts
Q41: Use the functions given by <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4588/.jpg"
Q78: Fairweather Corporation purchases merchandise on terms of
Q83: Suppose a paper mill in Quebec is