The pH of an acidic solution is a measure of the concentration of the acid particles in the solution, with smaller values of the pH indicating higher acid concentration. In a laboratory experiment, the pH of a certain acid solution is changed by dissolving over-the-counter antacid tablets into the solution. In this experiment, the pH changes according to the equation 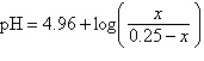 , where x is the number of grams of antacid added to the solution. What is the pH of the solution after the addition of 0.1 grams of antacid tablet?
, where x is the number of grams of antacid added to the solution. What is the pH of the solution after the addition of 0.1 grams of antacid tablet?
Definitions:
Business Firm
An organization engaged in commercial, industrial, or professional activities, aiming to generate profits by offering goods or services.
Taxes
Mandatory monetary levies enforced by a government on people or organizations to support public spending.
Price
The amount of money expected, required, or given in exchange for something else.
Gallons
A unit of volume measurement used primarily in the United States for liquids, equal to 3.785 liters.
Q1: rate used to discount projected merger cash
Q8: A price-taking firm in the short run
Q21: Refer to Figure 9-2. The short-run supply
Q22: Find the domain of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4588/.jpg" alt="Find
Q33: Match the equation with its graph. <img
Q47: Find all solutions to the following equation.
Q59: If <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4588/.jpg" alt="If is
Q61: Solve the system of linear equations <img
Q73: Find the standard form of the equation
Q107: one of your firm's customers is "stretching"