The volume V (in liters) of a certain mass of gas is related to its pressure P (in millimeters of mercury) and its temperature T (in degrees Kelvin) by the law 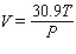 Compute
Compute 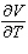 and
and 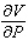 when T = 260 and P = 700.
when T = 260 and P = 700.
Definitions:
Population Variation
Differences or variations within a population, which can be due to genetic factors, environmental influences, or both.
P-values
In statistics, a measure that helps determine the significance of results from a hypothesis test, indicating the probability of observing the obtained results assuming the null hypothesis is true.
P-value
A statistical measure that helps in determining the significance of the results obtained from a hypothesis test, indicating the probability of observing the results by chance.
Statistical Significance
A result is said to be statistically significant if the computed p-value is less than some prescribed number, usually 0.05.
Q2: If a transportation problem has four origins
Q9: Evaluate the following improper integral whenever it
Q28: In a transshipment problem, shipments<br>A)cannot occur between
Q33: Find the indefinite integral. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7866/.jpg" alt="Find
Q42: Find the volume of the solid bounded
Q58: Use a double integral to find the
Q156: In a survey it was determined that
Q183: Find the area of the region under
Q221: Find the area of the region under
Q269: Because of the increasingly important role played