The volume V (in liters) of a certain mass of gas is related to its pressure P (in millimeters of mercury) and its temperature T (in degrees Kelvin) by the law 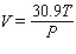 Compute
Compute 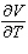 and
and 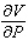 when T = 260 and P = 700.
when T = 260 and P = 700.
Definitions:
Labor Force
The cumulative count of individuals who either have a job or are seeking work actively.
Business Cycle Theories
Various economic theories that attempt to explain the causes and dynamics of the business cycle, including expansions and contractions in economic activity.
Endogenous
Originating from within an economic model or system; refers to factors that are explained by the internal workings of the model.
Exogenous
Factors or variables that are external to the economic model and not determined within the model itself.
Q2: The amount that the objective function coefficient
Q2: In a multiple channel system<br>A)each server
Q16: Find the volume of the solid bounded
Q17: A physical model that does not have
Q28: Minimize the function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7866/.jpg" alt="Minimize the
Q43: The annual sales (in billions of dollars)
Q81: Find the critical point(s) of the function.Then
Q112: Determine whether the given function is a
Q187: Evaluate the following improper integral whenever it
Q200: Find the critical point(s) of the function.Then