A buffer solution contains H2CO3 and HCO3, resulting in the equilibrium shown by the reaction below. According to this reaction and LeChatelier's principle, what happens when acid (H3O+) is added to this buffer solution? 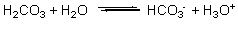
Definitions:
Chromosomes
Structures within cells that contain genetic information in the form of DNA, crucial for inheritance and cell division.
S Phase
Stage in interphase of the cell cycle during which DNA and other chromosomal constituents are synthesized. Compare with G1 phase and G2 phase.
Metaphase
A stage of cell division where chromosomes line up at the cell's equator before being separated into each of the two new cells.
Prophase
The first stage of cell division in both mitosis and meiosis, characterized by the condensation of the chromatin and the formation of the mitotic spindle.
Q2: The ionic compound CaCO<sub>3</sub> has a formula
Q2: Which of the following cases is not
Q3: Steven invested $25,000 as a limited partner
Q4: Which of the following statements accurately describes
Q5: Which of the following rules regarding the
Q37: The principal difference between a merchandising and
Q64: Methadone, a drug used to aid in
Q65: Which of the following diagrams best illustrates
Q68: Which of the following compounds is a
Q99: The following figure illustrates the action of