The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. What will happen when a small amount of base (OH) is added to the HF/F buffer? 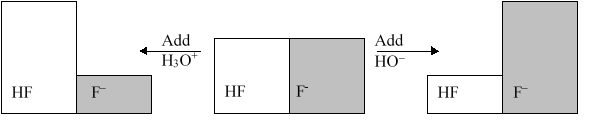
Definitions:
Bayesian Theorem
A formula that describes the probability of an event, based on prior knowledge of conditions that might be related to the event.
Posterior Probabilities
Revised probabilities of events based on additional information.
Independent Events
Two events A and B where P(A ∣ B) = P(A) or P(B ∣ A) = P(B); that is, the events have no influence on each other.
Probabilities
The quantification of an event's occurrence likelihood, represented as a figure from 0 to 1.
Q2: An investor has $50,000 to invest as
Q6: Based on the premise of the capitalization
Q8: Which of the following solutions has the
Q8: The steps for performing stoichiometry calculations are
Q31: The boxed species in the following reaction
Q39: In the body, glucose is broken down
Q54: A person receiving IV fluids must always
Q69: Converting a cis isomer to a trans
Q95: When naming in the IUPAC system, the
Q135: Below are several chemical equations, each labeled