Multiple Choice
The buffering system of the blood is shown below. Which of the following equations represents the reaction that occurs when hydroxide is added to this buffer? 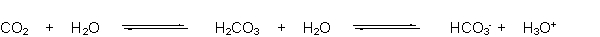
Definitions:
Related Questions
Q4: Sam wishes to purchase Kitchen Cabinets, Inc.
Q5: Which of the following rules regarding the
Q7: Consider solutions A and B separated by
Q12: Which of the following statements best describes
Q27: What change of phase is represented by
Q52: Calculate the molecular mass of aspirin, C<sub>9</sub>H<sub>8</sub>O<sub>4</sub>.<br>A)
Q65: What is the molecular geometry of the
Q77: The neutralization reaction of potassium hydrogen carbonate
Q120: Which of the following reactions is NOT
Q129: Cost of goods manufactured in a manufacturing