In the acid-base reaction between ammonia and water, which of the following substances are present at equilibrium? 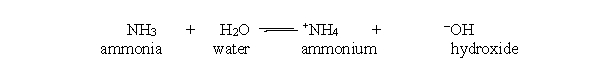
Definitions:
Convert
To change something into a different form, function, or state.
Divisor
A number by which another number is to be divided.
Numerical Expression
A mathematical statement that includes numbers, operations, and sometimes variables, but does not contain an equality or inequality sign.
Designated Whole Number
A specific, named integer that has been allocated for a particular purpose or identified in a particular context.
Q2: Mandy is a 40% partner in The
Q5: With regard to debt and equity securities,
Q6: Which of the following is not one
Q22: Dolan Company's accounting records reflect the
Q23: Which of the following statements does NOT
Q32: Why must a SCUBA diver ascend slowly
Q48: A _ has components that are evenly
Q50: Which of the following statements best describes
Q75: Which of the following substances are NOT
Q105: What is the pH of a solution