In the acid-base reaction between ammonia and water, which of the following statements best describes the concentration of ammonia and ammonium at equilibrium? 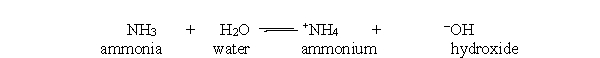
Definitions:
Throughput Time
The total time it takes for a unit to go through production from the start to the end, including both processing and waiting times.
Fill Orders
The process of completing a customer's purchase order by preparing and dispatching the ordered goods.
Delivery Cycle Time
The total time taken from receiving an order to delivering the product to the customer.
Fill Orders
The process of completing customer orders for goods or services.
Q3: Stuart Planter is a full-time teacher, and
Q3: Which of the following deductions are allowed
Q4: The shareholders of Parent Co. and Sub
Q4: Which of the following type of payment
Q7: Small Co. is for sale. The company
Q8: The Canadian tax system practices integration between
Q23: Which skeletal line structure best represents 2-butyne?
Q68: An arrow is pointing to the oxygen
Q71: ???A typical _ contains aggregates of atoms,
Q92: Managerial accounting reports can be described as<br>A)