A buffer solution contains H2CO3 and HCO3, resulting in the equilibrium shown by the reaction below. According to this reaction and LeChatelier's principle, what happens when acid (H3O+) is added to this buffer solution? 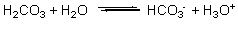
Definitions:
Interaction Effects
Occur when the effect of one independent variable on a dependent variable changes depending on the level of another independent variable.
Main Effects
The direct, independent impacts of individual independent variables on a dependent variable in the context of factorial designs.
Total Mean
The overall average of a set of numbers or measurements, calculated by summing all the values and dividing by the number of observations.
Total Mean
The overall average of a set of numerical values computed by summing them up and dividing by the count of numbers.
Q3: Blue Co. earned $80,000 in revenue and
Q7: Anthony is the sole shareholder of Glass
Q11: Which of the following describes the dissolution
Q31: Consider the two containers below, which are
Q32: Which statement best describes the difference(s) between
Q50: What is the IUPAC name of the
Q53: Air is primarily composed of nitrogen (594
Q55: This substance in the blood regulates the
Q58: In the acid-base reaction between ammonia and
Q76: Which of the following skeletal line structures