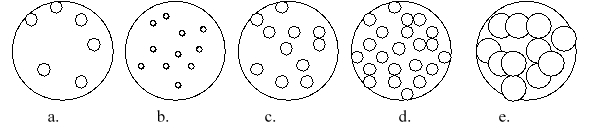
Imagine that you have a beaker of gas molecules. A small volume of the gas in the beaker is enlarged so you can see the gas particles. If all of the gas in the beaker A is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which magnified view best represents what the gas would look like? 
Definitions:
Q12: Consider the two containers below, which are
Q13: Which of the following diagrams illustrates an
Q16: To accomplish the change of phase shown
Q23: Which of the following pictures best represents
Q34: In which of the following reactions is
Q64: Methadone, a drug used to aid in
Q69: In general, how many bonds does a
Q77: Which isotope in Zirconium has the fewest
Q104: Which of the following biomolecules provides fuel
Q136: What is the most likely connectivity of