The reaction between acetic acid (CH3COOH) and methanol (CH3OH) is given below, followed by a list of changes that could be made to the reaction. Which of these changes will result in the equilibrium shifting to the left? 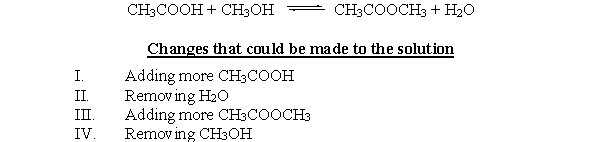
Definitions:
Fixed Input
A production factor that remains unchanged regardless of the level of output in the short run.
Marginal Product
The additional output generated by employing one more unit of a particular input, keeping other inputs constant.
Units of Labor
Measurements used to quantify the work input by labor forces, often referring to hours worked or number of workers.
Q21: Drinking water from a well is discovered
Q22: Which of these molecules contain(s) a secondary
Q29: Which of the following changes is not
Q47: Water (H<sub>2</sub>O) has a higher boiling point
Q49: A spirometer is used in indirect calorimetry.
Q53: During fatty acid oxidation, a fatty acid
Q62: Which of the following statements is the
Q95: Which of these molecules is the least
Q102: Which atom is in its ground state?
Q112: The reaction of water with ammonia is