Why does the lattice of Li2O below show more ions than the formula unit? 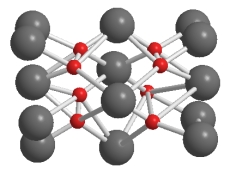
Definitions:
Incidence
The occurrence, rate, or frequency of a disease, crime, or something else undesirable.
Dental Disease
A range of conditions that affect the health of the teeth and gums, including cavities, gum disease, and oral cancer.
Correlation Coefficient
A measure of correlation that ranges in value from –1.00 to +1.00.
Meta-Analysis
A set of techniques for combining data from a number of related studies to determine the explanatory strength of a particular independent variable.
Q4: How many electrons are in the valence
Q7: The periods are the _ of the
Q8: Which bond in ATP is referred to
Q34: The following is step 8 of the
Q35: Which electron shell is closest to the
Q37: Why are there many different ways to
Q43: Which atom has the highest electronegativity in
Q60: Why does the HIV virus need a
Q74: ATP-synthase, an enzyme that catalyzes the phosphorylation
Q124: The following ionic formula is not valid.