Multiple Choice
Does propane or octane (C8H18) exhibit stronger dispersion forces? 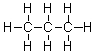
Definitions:
Related Questions
Q19: The arrow is pointing to a(n)<br>A) <img
Q21: Which of the following values is Avogadro's
Q30: According to the following graph of activity
Q42: What is the IUPAC name of the
Q48: _ are neutral.<br>A) Protons<br>B) Neutrons<br>C) Electrons<br>D) Protons
Q62: Which of the following structures is a
Q82: One serving (45 g) of dry wild
Q132: Below are two ions (not drawn to
Q139: In addition to single bonds, which of
Q159: Which of the following covalent molecules is