Why does the lattice of Li2O below show more ions than the formula unit? 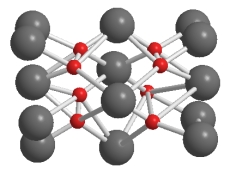
Definitions:
High Blood Pressure
A condition where the blood moves through the arteries at a higher pressure than what is considered normal, potentially leading to health issues.
Exercise
Physical exertion or activity done to maintain or improve health and fitness.
Sexual Expression
The ways in which individuals convey their sexuality or sexual feelings, which can vary widely across culture, time, and individual preference.
Heterosexual Women
Women who are romantically and sexually attracted to men.
Q1: What is the relationship between the following
Q2: What happens to the speed of molecules
Q2: Which of the following is the best
Q13: Which of the following statements about glycolysis
Q31: The energy diagram below is for an
Q43: Which of the following is a potential
Q50: Two molecules of carbon dioxide are released
Q78: One of the steps of glycolysis is
Q86: What is meant by the following symbols?
Q103: Which of the following elements has a