Is the aldehyde oxidized or reduced during the following reaction, and how can you tell? 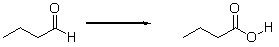
Definitions:
Oral Cancer
A type of cancer that develops in the tissues of the mouth or throat, often linked to tobacco use, heavy alcohol use, or the presence of human papillomavirus infection.
Dietary Nutrients
Essential chemical compounds found in food that the body needs to function properly, including vitamins, minerals, proteins, carbs, and fats.
Randomized
Refers to the process of making something random, especially in scientific studies to distribute participants or conditions in a way that is determined by chance.
Controlled Trials
A scientific method used to test the effectiveness and safety of interventions by comparing outcomes between a controlled group and an experimental group.
Q8: The Fischer projection for fructose is shown
Q17: In which of the following structures is
Q35: Beyond the Boardroom Corporation sold used equipment
Q42: On an indirect method statement of cash
Q49: How many mg of Tylenol should be
Q59: What type of reaction is this? <img
Q74: The bar graph below shows the percentage
Q83: During replication, the DNA is unwound by
Q101: What type of secondary structure is in
Q104: Which accounting principle directs the depreciation process?<br>A)