Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the standard enthalpy change ?H for the hydrogenation of ethyne (acetylene) to ethane. 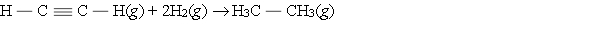
Definitions:
Live In Poverty
The condition of someone who lacks sufficient income or material possessions for a standard of living considered acceptable in society.
Absolute Level
A term used to describe the total magnitude of a quantity, not compared or relative to other measurements.
Population
The total number of individuals inhabiting a particular area or country.
Equal Distribution
The concept of distributing resources or wealth evenly across a society or group, aiming for fairness and equity.
Q5: Computers that process data from sensors as
Q5: Which of the following atoms will be
Q15: Supply-chain automation<br>A) streamlines organizations by eliminating transactional
Q20: In 2005 Sony BMG Music Entertainment made
Q32: Hydrogen peroxide was catalytically decomposed and
Q35: Which statement best supports the conclusion that
Q41: The melting points of metals are only
Q48: O'Leary suggests that there is a continuum
Q54: Electron affinities become increasingly positive toward the
Q88: The ideal gas law tends to become