Identify the element of Period 2 which has the following successive ionization energies, in kJ/mol. 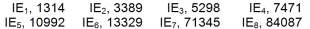
Definitions:
Proactive Interference
The phenomenon where previously learned information interferes with the recall of newer information.
Retroactive Interference
A cognitive phenomenon where newly learned information interferes with the recall of previously learned information.
Korsakoff's Syndrome
A chronic memory disorder caused by severe deficiency of thiamine (vitamin B1), most often associated with alcoholism.
Short-Term Memory
The part of the memory system where information is stored for a short duration, typically seconds to minutes.
Q5: Which of the following atoms will be
Q12: The standard state of a substance in
Q13: What benefit does a company gain by
Q16: In the fourth century the codex replaced
Q16: Which of the following elements is the
Q35: Select the classification for the following
Q46: Select the correct electron configuration for Cu
Q47: The orientation in space of an atomic
Q73: Hund's rule is used to predict the
Q92: Magnesium fluoride is used in the ceramics