Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 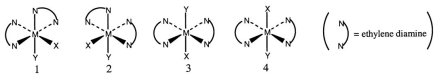 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?
Definitions:
Related Questions
Q14: When an alkali metal combines with a
Q28: Cesium-134 is a <span class="ql-formula"
Q42: According to valence bond theory, what would
Q77: Chromium metal is electroplated from acidic aqueous
Q79: Ethanol, C<sub>2</sub>H<sub>5</sub>OH, is being promoted as
Q83: How many milliliters of 1.58 M
Q84: A) How many unpaired 3d electrons will
Q90: A buffer is prepared by adding 150
Q93: . If the partial pressure of argon
Q97: The substance, KClO<sub>3</sub>, is a strong oxidizer