When the following redox equation is balanced with smallest whole number coefficients, the coefficient for the hydrogen sulfate ion will be ______. 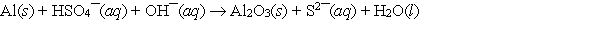
Definitions:
Misrepresenting Reality
The act of presenting false or distorted information as truthful, often to manipulate public perception or opinion.
Culture Wars
Conflicts between groups with differing beliefs, values, or lifestyles, often reflected in political, religious, or social debates.
Traditionalist Values
Traditionalist values refer to the beliefs and practices that emphasize preserving historical norms and cultural heritage.
Conservative Values
Conservative Values are principles that emphasize tradition, stability, and respect for authority, often associated with right-leaning political ideologies.
Q2: In both of the following reactions, a
Q6: The nuclide Pb-210 undergoes three successive decays
Q10: Transition elements from the left side of
Q32: The chlor-alkali process produces chlorine, Cl<sub>2</sub>(g), in
Q40: What is the value of K<sub>b</sub> for
Q44: How many moles of ions are released
Q45: Ferric oxide is used as a pigment
Q52: Which one of the following statements about
Q80: Zinc acetate is used in preserving wood
Q87: A) Give the names of the following