The redox reaction of peroxydisulfate with iodide has been used for many years as part of the iodine clock reaction which introduces students to kinetics. If E cell = 1.587 V and E of the cathode half-cell is 0.536 V, what is E of the anode half-cell? 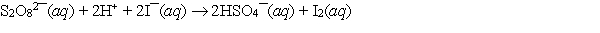
Definitions:
Population Proportion
A statistical measure that represents the fraction or percentage of individuals in a specific group within the larger population.
Achievement Motivation
A psychological feature that drives individuals to pursue and attain goals or accomplishments.
McClelland
David McClelland, a psychologist known for his work on motivation, particularly the theory of needs which includes the need for achievement, power, and affiliation.
High Standards
Expectations or criteria that are considerably above average in terms of level, quality, or performance.
Q26: Describe, and give reasons for, the fundamental
Q28: A) Explain or define what is meant
Q37: How many unpaired electrons are there in
Q40: Carbon atoms in the carbon cycle spend
Q53: The radioisotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt=" The
Q61: Sodium chlorate is used as an oxidizer
Q66: A much-studied cell in electrochemistry has the
Q82: Given: H<sub>2</sub>O(l) <span class="ql-formula" data-value="\rightarrow"><span
Q86: A diprotic acid H<sub>2</sub>A has K<sub>a1</sub> =
Q107: The solubility of calcium chromate is 1.56