Examine the following half-reactions and select the strongest reducing agent among the species listed. 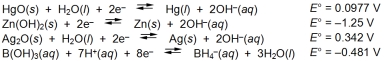
Definitions:
Sales Manager
A professional responsible for leading and directing a sales team to meet or exceed sales targets.
Manufacturing Costs
The total expenses involved in the process of producing goods, including raw materials, labor, and overhead costs.
Financing
The process of providing or obtaining the funds necessary for investment, expansion, or operations.
Repayments
Amounts of money paid back to lenders or creditors in partial or full settlement of a loan or debt.
Q8: Calcium fluoride, CaF<sub>2</sub>, is a source of
Q20: Aqueous potassium iodate (KIO<sub>3</sub>) and potassium
Q24: For real gases, PV < nRT, always.
Q35: A certain process has <span
Q41: Sulfur dioxide reacts with chlorine to
Q50: Increasing the concentrations of the components of
Q52: Which, if any, of the following acids
Q58: What will be the effect of adding
Q65: Select the correct relationship among the concentrations
Q76: Hydrated metal ions in aqueous solution can