Calculate E cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. 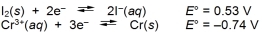 Overall reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I¯(aq)
Overall reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I¯(aq)
Definitions:
Seasoned Equity Offering
Refers to the process of a publicly-traded company issuing additional shares of stock to investors after the initial public offering.
Q1: The main effect of the biosphere on
Q6: Which of the following coordination numbers applies
Q16: Assuming that the total volume does not
Q22: Use the given data at 298
Q32: What is the E <span class="ql-formula"
Q36: What is the pK<sub>a</sub> for the acid
Q54: Examine the following half-reactions and select the
Q67: Which one of the following ionic compounds
Q103: A solution is prepared by dissolving 20.0
Q111: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution