What is the value of the equilibrium constant for the cell reaction below at 25 C?
E cell = 0.30 V Sn2+(aq) + Fe(s) 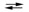 Sn(s) + Fe2+(aq)
Sn(s) + Fe2+(aq)
Definitions:
Stock At A Premium
Stock at a premium refers to shares that are sold for a price higher than their par value, often reflecting the issuing company's perceived value and growth prospects.
Income Statement
A financial statement that shows a company's revenues and expenses over a specified period, culminating in the net income or loss for that period.
Retained Earnings
Profits that a company keeps or reinvests rather than distributing to shareholders as dividends.
Cash Available
The total amount of cash or liquid funds a company or individual has accessible for immediate use.
Q5: Which of the following substances has the
Q8: Exposure to 10 nCi for 10 minutes
Q17: What is the value of K<sub>b</sub> for
Q20: The most abundant element in the Earth's
Q38: Constitutional (structural) isomers have the same empirical
Q71: Calculate the oxidation number of sulfur in
Q71: A certain transition element has the stable
Q82: The compound, NaH<sub>2</sub>PO<sub>4</sub>, is present in many
Q83: Briefly, explain the relationship between the rad
Q97: Identify all the spectator ions in