What is the value of the equilibrium constant for the cell reaction below at 25 C?
E cell = 0.61 V 2Cr(s) + 3Pb2+(aq) 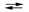 3Pb(s) + 2Cr3+(aq)
3Pb(s) + 2Cr3+(aq)
Definitions:
Intelligence Information
Information that has been collected, analyzed, and used for the purpose of making informed decisions, often within a security or strategic context.
External Environment
Factors outside of an organization that can impact its operations and success, including economic, social, and political conditions.
Organization's Boundaries
The defined limits that distinguish the activities, responsibilities, and jurisdictions of an organization from others.
Management Information System
An organized system for collecting, storing, analyzing, and disseminating information for the purpose of facilitating management decision-making processes.
Q15: Examine the following half-reactions and select the
Q21: When nitrogen undergoes atmospheric fixation, it enters
Q22: Calcium hydroxide is used in mortar, plaster
Q24: Reduction is the loss of electrons.
Q39: Aqueous solutions of phosphoric acid and sodium
Q43: Which relationship best describes <span
Q49: Aluminum sulfate, Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>, is used in tanning
Q56: A solution is prepared by adding 500
Q70: One mole of methane (CH<sub>4</sub>) contains a
Q113: A buffer is to be prepared by