The following half-reactions occur in the mercury battery used in calculators. If E 1U1B1cell = 1.357 V, calculate the equilibrium constant for the cell reaction at 25 C. (Assume the stoichiometric coefficients in the cell reaction are all equal to 1.) HgO(s) + H2O(l) + 2e¯ 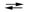 Hg(l) + 2OH¯(aq)
Hg(l) + 2OH¯(aq)
ZnO(s) + H2O(l) + 2e¯ 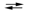 Zn(s) + 2OH¯(aq)
Zn(s) + 2OH¯(aq)
Definitions:
Reactive Depression
A type of depression triggered by a significant stressful life event or situation.
Stressful Events
Incidents or situations that trigger a state of emotional or physical tension, often requiring adjustment or coping mechanisms from the individual.
Endogenous Depression
Endogenous Depression is a subtype of major depressive disorder thought to occur due to internal biochemical imbalances rather than external stressors.
Major Depressive Episode
A period characterized by intense feelings of sadness, hopelessness, and a lack of interest or pleasure in activities, lasting at least two weeks.
Q20: Ammonium chloride is used as an electrolyte
Q28: A 10.0-mL sample of 0.75 M CH<sub>3</sub>CH<sub>2</sub>COOH
Q38: Electrometallurgy uses _ to separate a metal
Q56: The M<sup>2+</sup> ions of the first transition
Q67: Which one of the following ionic compounds
Q69: Aluminum oxide, Al<sub>2</sub>O<sub>3</sub>, is used as a
Q84: A) How many unpaired 3d electrons will
Q85: Select the pair of substances in which
Q93: Consider the dissolution of MnS in water
Q96: The lead-acid battery is an example of