Consider the reaction of iodine with manganese dioxide:
3I2(s) + 2MnO2(s) + 8OH¯(aq) 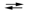 6I¯(aq) + 2MnO4¯(aq) + 4H2O(l)
6I¯(aq) + 2MnO4¯(aq) + 4H2O(l)
The equilibrium constant for the overall reaction is 8.30* 10¯7. Calculate G for the reaction at 25 C.
Definitions:
Remedy
A means of legal reparation or correction provided to a party who has suffered a loss or injury.
Commercial Reasonableness
The standard by which business actions are judged, based on what a reasonable person would consider fair and appropriate under the circumstances.
Consequential Damages
Refers to the secondary effects or financial losses that occur as a result of a breach of contract, beyond the immediate scope of the agreement.
Substitute Goods
Products or services that can serve as replacements for one another, satisfying the same consumer need.
Q5: One mole of O<sub>2</sub> has a mass
Q6: A row of the periodic table is
Q9: Analysis of a carbohydrate showed that it
Q20: A reaction has a positive value
Q32: Which one of the following is a
Q39: In an acid-base (neutralization) reaction the indicator
Q50: The substance NaNO<sub>3</sub> is considered<br>A) a weak
Q52: Which of the following is most soluble
Q67: Use the following information to calculate the
Q74: Silicon, which makes up about 25% of