Consider the reaction of iodine with manganese dioxide:
3I2(s) + 2MnO2(s) + 8OH¯(aq) 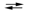 6I¯(aq) + 2MnO4¯(aq) + 4H2O(l)
6I¯(aq) + 2MnO4¯(aq) + 4H2O(l)
The equilibrium constant for the overall reaction is 8.30 *10¯7. Calculate E cell for the reaction at 25 C.
Definitions:
Fields of Experience
The various backgrounds, knowledge areas, and skills that individuals bring to an organization or a team.
Slogan Translation
The process of adapting a slogan from one language to another while preserving its meaning, tone, and impact to appeal to different linguistic and cultural groups.
Encoding
The process of converting ideas or messages into words, symbols, or images for communication.
Communication Process
The series of steps through which information is exchanged between individuals or groups, including sending, receiving, and interpreting messages.
Q5: All Brønsted-Lowry bases contain the hydroxide ion,
Q23: After 4 half-lives, the fraction of a
Q28: Which relationship or statement best describes
Q30: Consider the balanced equation: Al<sub>2</sub>S<sub>3</sub>(s) +
Q73: A solution is prepared by adding 0.10
Q74: Octahedral complexes can exhibit geometric, optical and
Q79: Hydrogen sulfide decomposes according to the
Q88: When a chemical system is at equilibrium,<br>A)
Q100: What is the pH of a 0.0125
Q108: What is the pH of a buffer