Calculate G for the reaction of ammonia with fluorine. 
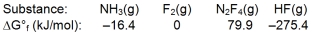
Definitions:
Social Security Act
A law enacted to provide for the general welfare by establishing a system of federal old-age benefits, and by enabling states to make more adequate provision for aged persons, blind persons, dependent and crippled children, maternal and child welfare, public health, and the administration of their unemployment compensation laws.
Employee Retirement Income Security Act
A federal law that sets minimum standards for most voluntarily established retirement and health plans in private industry to provide protection for individuals in these plans.
Family and Medical Leave Act
A U.S. law that provides employees with up to 12 weeks of unpaid, job-protected leave per year for certain family and medical reasons.
Consolidated Omnibus Budget Reconciliation Act
A federal law in the United States that provides individuals and their families the right to continue health care coverage under the group plan for limited periods under certain conditions.
Q2: In complexes of transition metals, the maximum
Q8: Exposure to 10 nCi for 10 minutes
Q17: For a chemical reaction to be
Q17: The solubility of magnesium phosphate is 2.27
Q30: A chemical reaction has <span
Q54: The reaction CH<sub>3</sub>NC(g) <span class="ql-formula"
Q54: Electrolysis is used as the last step
Q58: Nitrogen dioxide decomposes according to the
Q61: The water-gas shift reaction plays an important
Q71: The entropy of one mole of oxygen