Solved
Consider the Figure Below Which Shows G For a Chemical Process Plotted Against Absolute Temperature
Multiple Choice
Consider the figure below which shows G for a chemical process plotted against absolute temperature. 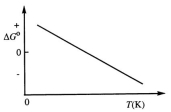 From this plot, it is reasonable to conclude that:
From this plot, it is reasonable to conclude that:
Definitions:
Related Questions
Q4: What is the name of Na<sub>2</sub>O?<br>A) disodium
Q5: What elements are alloyed to make stainless
Q41: An aqueous solution is considered to be
Q49: The radioisotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt=" The
Q53: A voltaic cell has a standard cell
Q57: A primary battery is one which can
Q69: Calculate the potential of a voltaic
Q76: Radioactive decay follows zero-order kinetics.
Q78: Iron (III) chloride hexahydrate is used as
Q82: When metal A is placed in