Consider the dissolution of MnS in water (Ksp = 3.0 * 10¯14) . 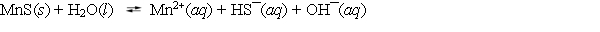 How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
Definitions:
Its Direction
Refers to the course along which someone or something moves or is aimed to move.
Its Strength
Refers to the inherent capabilities or advantages possessed by an entity, product, or concept that contribute to its effectiveness or success.
Its Magnitude
Describes the size, extent, or importance of something, often used to express the degree of impact or significance.
Cognitive Dissonance
is the psychological discomfort experienced when holding two or more contradictory beliefs, ideas, or values.
Q10: Sulfur trioxide can undergo decomposition according
Q25: What is the value of K<sub>a</sub> for
Q25: Alloying a metal is done to<br>A) make
Q25: A 20.0-mL sample of 0.30 M HClO
Q29: The ammonium ion, NH<sub>4</sub><sup>+</sup>, is a weak
Q35: An elementary reaction is a simple, one-step
Q57: When the reaction A <span class="ql-formula"
Q60: . If the compositions of the two
Q76: Hydrated metal ions in aqueous solution can
Q103: The molecular formula of a compound provides