A student adds 0.1 mol of oxalic acid and 0.1 mol of sodium dihydrogen phosphate to enough water to make 1.0 L of solution. The following equilibrium is established with the concentrations of the products greater than the concentrations of the reactants. Which of the statements about the equilibrium system is correct? 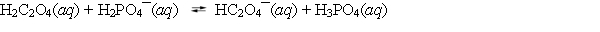
Definitions:
Poverty Threshold
The minimum level of income deemed necessary to achieve an adequate standard of living in a given country or region; a measure used to identify those in poverty.
Market Situation
The current condition of a marketplace, determined by factors like supply, demand, and economic indicators.
Market Value
The current price at which an asset or service can be bought or sold in a marketplace.
Family History
The study of the lineage and history of families, including genealogy, inherited traits, and shared heritage.
Q51: Sulfur trioxide is the anhydride of sulfuric
Q55: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q58: Select the correct type for the following
Q68: In going from room temperature (25.0
Q70: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q80: The term "microstate" refers to the energy
Q104: Including cyclic compounds, how many possible isomers
Q107: Which element forms compounds which are used
Q111: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q112: Select the correct type for the