The equilibrium constant, Kp, for the reaction 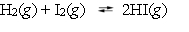 is 55.2 at 425 C. A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
is 55.2 at 425 C. A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
Definitions:
Pattern Of Classification
A method of organizing or categorizing information based on shared characteristics or criteria.
Chemistry Majors
University or college students who specialize in the study of chemistry, focusing on the properties, composition, and transformations of matter.
Class Schedules
Timetables that outline the times and locations of classes within an educational institution.
Organizational Pattern
A framework or strategy used in writing or speech to arrange ideas and information in a coherent, logical manner.
Q5: Which of the following features do cyclohexene
Q8: If a strong acid such as HCl
Q16: Which one of the following statements relating
Q24: If the activation energy of a reaction
Q36: In an exothermic reaction,<br>A) the forward reaction
Q47: In a chemical reaction, if the starting
Q75: For each of the following names, write
Q77: The second law of thermodynamics tells us
Q81: What is the mass-action expression, Q<sub>c</sub>, for
Q86: The decomposition of dinitrogen pentaoxide to