A mixture of 0.600 mol of bromine and 1.600 mol of iodine is placed into a rigid 1.000-L container at 350 C. 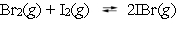 When the mixture has come to equilibrium, the concentration of iodine monobromide is 1.190 M. What is the equilibrium constant for this reaction at 350 C?
When the mixture has come to equilibrium, the concentration of iodine monobromide is 1.190 M. What is the equilibrium constant for this reaction at 350 C?
Definitions:
Societal Issues
Challenges or problems that affect a large portion of society, which may include subjects like poverty, education, and environmental degradation.
Marketing Ethics
The area of applied ethics dealing with the moral principles behind the operation and regulation of marketing.
Environment
The sum of all external conditions and influences affecting the life, development, and survival of organisms, including natural, built, and social surroundings.
Corporate Social Responsibility
The commitment by businesses to behave ethically and contribute to economic development while improving the quality of life of the workforce and their families as well as the local community and society at large.
Q26: A chemical reaction will reach equilibrium when
Q44: In the electrolysis of aqueous potassium nitrate
Q76: Predict the products for the following
Q84: All Brønsted-Lowry bases have at least one
Q88: Which of the following pairs is arranged
Q92: Which of the following aqueous solutions should
Q98: A solution of sucrose (sugar) in water
Q101: The compound, BaO, absorbs water and carbon
Q105: A solution of sodium acetate (CH<sub>3</sub>COONa) in
Q109: Select the correct name for this compound.