The following reaction, in CCl4 solvent, has been studied at 25 C. 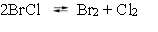
The equilibrium constant Kc is known to be 0.141. If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M, what is the equilibrium concentration of bromine?
Definitions:
Donee Beneficiary
A third party who benefits from a contract made between two other parties, particularly where the contract's intent is to gift something to the beneficiary.
Creditor Beneficiary
A third party that benefits from a contract in which one party promises to pay a debt owed to the third party by the other contract party.
Promisee
The party in a contract who is promised something by another party (the promisor) and stands to benefit from the fulfillment of that promise.
Novation
The act of replacing one obligation with another, which involves a new party or a new agreement and extinguishes the original contract.
Q8: A study of the decomposition reaction
Q47: A 0.100 m K<sub>2</sub>SO<sub>4</sub> solution has
Q57: What types of forces exist between molecules
Q65: What volume of concentrated (14.7 M) phosphoric
Q69: Sulfuryl chloride, SO<sub>2</sub>Cl<sub>2</sub>(g), decomposes at high temperature
Q74: Briefly list the features/properties common to all
Q74: 2-chloro-2,3-dimethylbutane will react with potassium hydroxide dissolved
Q79: Which one of the following quantities is
Q92: Which one of the following is a
Q94: element that lies in period 4?<br>A) Cr<br>B)