Magnesium hydroxide is used in several antacid formulations. When it is added to water it dissociates into magnesium and hydroxide ions. 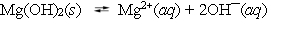 The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
Definitions:
Legal Structure
The framework within which a business operates, including its designation as a corporation, partnership, sole proprietorship, or other legal entity.
Barrier To Entry
Factors that make it difficult for new competitors to enter a market, such as high start-up costs or regulatory hurdles.
Price-Control Legislation
Laws enacted by a government to regulate the prices charged for goods and services in the market, often to protect consumers.
Invisible Hand Principle
The tendency of market prices to direct individuals pursuing their own interests to engage in activities promoting the economic well-being of society.
Q1: Which of the following affects the activation
Q49: The mass of a neutron is equal
Q53: Which of the following is true
Q75: Hydrogen peroxide, H<sub>2</sub>O<sub>2</sub>, is<br>A) used in the
Q82: Select the correct name for the following
Q85: Carbon-14 is a radioactive isotope which decays
Q87: Which of the following oxides will give
Q92: Which of the following elements has the
Q98: A solution is prepared by adding 100
Q99: A saturated solution of calcium hydroxide, Ca(OH)<sub>2</sub>,