Methanol can be synthesized by combining carbon monoxide and hydrogen. 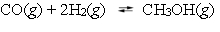 A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
Definitions:
Optimal Output
The level of production where a firm maximizes its profits or minimizes its losses.
Differentiated Products
Products that are distinguished from others through variations in quality, features, design, branding, or customer service.
Equilibrium Price
The market price at which the quantity of goods supplied is equal to the quantity of goods demanded.
Reaction Curve
A graph that shows how one player's optimal strategy choice depends on the strategy choice of another player.
Q3: The solubility of lead(II) chloride is 0.45
Q10: You are given pure samples of
Q20: In the gas phase, AlCl<sub>3</sub> exists as
Q32: Which one of the following is a
Q41: The halogens are<br>A) strong oxidizing agents.<br>B) strong
Q54: The reaction CH<sub>3</sub>NC(g) <span class="ql-formula"
Q59: Barium fluoride is used in embalming and
Q71: For the reaction A(g) + 2B(g)
Q80: Zinc acetate is used in preserving wood
Q97: Name the three component parts of a