Nitrogen dioxide can dissociate to nitric oxide and oxygen. 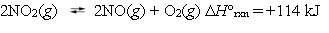 Under which reaction conditions would you expect to produce the largest amount of oxygen?
Under which reaction conditions would you expect to produce the largest amount of oxygen?
Definitions:
Equity Account
An account representing the owner's or shareholders' residual interest in the assets of the entity after deducting liabilities.
Stockholders
Stockholders are individuals or entities that own shares of stock in a corporation, thereby holding ownership interests and potentially benefiting from the company's profitability through dividends and stock price appreciation.
Preferred Stock
A class of ownership in a corporation that has a higher claim on assets and earnings than common stock, often providing dividends before common shares.
Stockholders' Equity
The ownership interest of shareholders in a company, calculated as total assets minus total liabilities.
Q7: A 20.0-mL sample of 0.30 M HBr
Q31: Write the mass-action expression, Q<sub>c</sub>, for the
Q40: In a fuel cell, an external source
Q46: Excluding cyclic compounds, how many possible isomers
Q58: You are required to determine the energy
Q84: Which of the following elements exists in
Q89: Carboxylic acids are weak acids.
Q93: A metal with a body-centered cubic lattice
Q95: Cinnamaldehyde (? = 132.15 g/mol) is
Q108: What is the pH of a buffer