The following reaction is at equilibrium at a pressure of 1 atm, in a closed container. 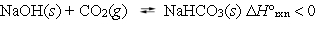 Which, if any, of the following actions will decrease the concentration of CO2 gas present at equilibrium?
Which, if any, of the following actions will decrease the concentration of CO2 gas present at equilibrium?
Definitions:
Machine Learning
A subset of artificial intelligence (AI) focusing on algorithms and statistical models that enable computers to perform tasks without explicit instructions by relying on patterns and inference.
Intelligent Systems
Intelligent systems refer to advanced computing systems that can learn, adapt, and perform tasks autonomously, often involving artificial intelligence technologies.
Mobile Text
Written communication sent or received through mobile devices, commonly known as text messages or SMS.
Scroll Down
An action typically performed on a digital device to view content not immediately visible on the screen.
Q1: For a diprotic acid H<sub>2</sub>A, the relationship
Q30: Both alkenes and alkynes exhibit geometric (cis/trans)
Q33: Aspirin is an effective and widely used
Q43: Which of the alkali metals has the
Q44: Which of the following statements about the
Q52: Ammonium iodide dissociates reversibly to ammonia
Q55: Potassium fluoride is used for frosting glass.
Q63: When identical particles pack in a simple
Q79: Which one of the following quantities is
Q81: Draw and name all stable molecules with